Summary:
Compared with conventional therapy for the management of acute wheezing episodes in young children with intermittent asthma and severe exacerbations, which consists of inhaled bronchodilators followed by the sequential addition of systemic corticosteroids, intervention with an inhaled corticosteroid (ICS) or leukotriene receptor antagonist (LTRA) at the onset of respiratory tract illness (RTI)-associated symptoms will not increase the proportion of episode-free days over the 12-month study period.
Design:
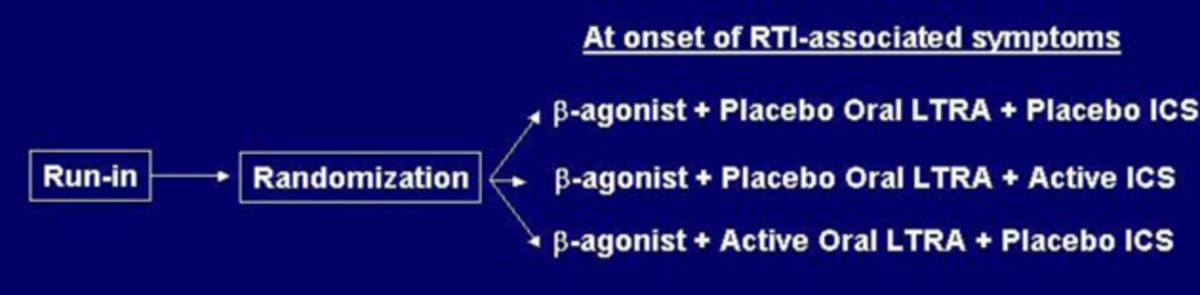
The AIMS design is a randomized, double-blind, placebo-controlled parallel comparison of three strategies directed at minimizing symptoms of wheezing during acute RTIs in children 12-59 months of age with histories of moderate-severe episodes of wheezing. There is a 2-week observation period to qualify and characterize a child, followed by randomization to one of three treatment groups and a 12-month follow-up period. When a child displays the first sign of RTI-associated symptoms, the parent/guardian administers one of the following treatment regimens for 7 days: (1) active ICS (Pulmicort Respules® 1.0 mg bid) and placebo LTRA; (2) active LTRA (Singulair 4 mg once daily, granules for children 12-23 months of age and chewable tablets for children 24-59 months of age) and placebo ICS; (3) placebo ICS and placebo LTRA. All children receive albuterol inhalation treatments four times daily while awake (plus as needed) for the first 48 hours followed by albuterol by inhalation on an as-needed basis. Additional rescue albuterol treatments may be administered on an as-needed basis, and oral steroids are available as well. The primary outcome variable is the proportion of episode-free days during the 12-month follow-up period. Secondary outcome variables include time to initiation of first course of oral steroids, total number of courses of oral steroids, duration and severity of lower respiratory tract symptoms, as reflected by the percentage of episode-free days and symptom scores respectively in the 14-day intervals following initiation of study medication, number of wheezing episodes, time to treatment failure, days missed from daycare, parental work and caregiver quality of life, number of unscheduled visits for acute wheezing episodes, and linear growth.
Population:
Childhood Asthma Research and Education (CARE) Network
Link:
Enrollment for AIMS began in February 2004 with a target sample size of 225 randomized children. Enrollment was completed in November 2004 with 238 randomized children, and the final patient visits occurred in November 2005. The results indicated that the addition of montelukast or budesonide at the early signs of RTIassociated symptoms did not differ from albuterol alone with respect to EFDs, growth, adverse effects, and the total number of courses of oral corticosteroids, urgent care visits, and emergency room visits. The addition of montelukast or budesonide decreased the severity of symptoms during acute episodes when compared to albuterol alone. The major manuscript is in press at JACI:
Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF Jr, Sorkness CA, Bloomberg GR, Morgan WJ, Paul I, Guilbert TW, Krawiec M, Covar R, Larsen G, Moss MH, Chinchilli VM, Taussig LM. Strunk RC. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol 2008;122:6:1127-1135.e8 PMID: 18973936.

