Summary:
In children 6-18 years of age, whose asthma symptoms are not acceptably controlled by low dose inhaled corticosteroid (ICS) therapy, the following three step-up therapies will not differ with respect to asthma control: (1) doubling the dose of ICS; (2) adding a long-acting beta-agonist and not increasing the ICS dose; (3) adding a leukotriene receptor antagonist and not increasing the ICS dose.
Design:
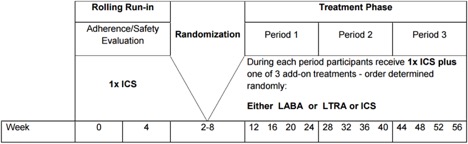
BADGER is a 56-week randomized, double-blind, three-treatment, three-period cross-over trial that will evaluate the differential improvement in control that is achieved following three separate treatment interventions in children whose asthma is not acceptably controlled on a low dose of ICS (per NAEPP guidelines). All participants will enter an 8-week run-in period during which time they will receive a dose of 1x ICS (fluticasone 200 μg/day). During this 8-week time period, running 2-week averages to establish the lack of acceptable asthma control will be calculated. Thus, a child could qualify for randomization at any time during this 8-week run-in period. This approach should maximize both patient safety and successful enrollment. Children will continue to receive 1x ICS during the entire treatment phase. During each period of the treatment phase, they also will receive one add-on therapy in the form of LABA, LTRA or additional 1x ICS. The order of the add-on therapy assignment will be determined by randomization into one of six treatment sequences (order determined randomly). Each treatment period will be 16 weeks in length; the initial 4 weeks of each period will be considered to be the washout period for the previous treatment. The primary outcome measures will be frequency of asthma exacerbations, asthma control days, and FEV1.
Population:
Childhood Asthma Research and Education (CARE) Network
Link:
Enrollment for BADGER began in February 2007 and 182 children were randomized into the six treatment sequences. The final patient visits are anticipated to occur in June 2010. A differential response occurred in 161 of 165 patients who were evaluated (P<0.001). The response to LABA step-up therapy was most likely to be the best response, as compared with responses to LTRA step-up (relative probability, 1.6; 95% confidence interval [CI], 1.1 to 2.3; P = 0.004) and ICS step-up (relative probability, 1.7; 95% CI, 1.2 to 2.4; P = 0.002). Higher scores on the Asthma Control Test before randomization (indicating better control at baseline) predicted a better response to LABA step-up (P = 0.009). White race predicted a better response to LABA step-up, whereas black patients were least likely to have a best response to LTRA step-up (P = 0.005). However, many children had a best response to ICS or LTRA step-up therapy, highlighting the need to regularly monitor and appropriately adjust each child’s asthma therapy. The major publication appeared in NEJM:
Lemanske Jr RF, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, Covar RA, Guilbert TW, Larsen G, Morgan WJ, Moss MH, Spahn JD, Taussig LM. Step-up therapy for children with uncontrolled asthma while receiving inhaled corticosteroids. NEJM 2010; 362:975-985. PMID: 20197425. PMCID:PMC2989902.

