Summary:
In children with mild to moderate persistent asthma, there are no differences within individual subjects in the magnitude of response as determined by improvement in FEV1, for inhaled fluticasone propionate and montelukast when they are administered at recommended doses.
Design:
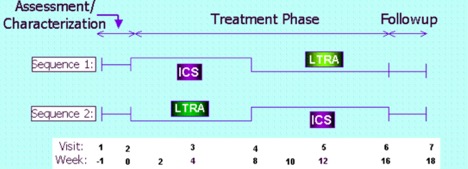
Children with mild to moderate persistent asthma, ages 6-17 years, were randomized to one of two crossover sequences including 8 weeks of ICS (fluticasone propionate 100 μg bid), and 8 weeks of LTRA (montelukast 5- 10 mg nightly depending on age), in a multi-center, double-masked, 18-week trial. Children must have exhibited reversible airflow obstruction (≥ 12% improvement in FEV1 following the maximal bronchodilator testing procedure with albuterol MDI) or a methacholine PC20 ≤ 12.5 mg/ml to qualify for study entry. There was no washout period between treatment administrations, so the first four weeks of the second treatment administration served as a pseudo-washout. Response was assessed by improvement in FEV1 and examined for relationships to baseline asthma phenotype-associated biomarkers.
Population
Childhood Asthma Research and Education (CARE) Network
Enrollment for CLIC began in January 2002 and the last patient visits occurred in March 2003. There were 144 randomized children and 126 children completed the trial. Defining response as improvement in FEV1 ≥ 7.5%, 17% of 126 participants responded to both medications, 23% to fluticasone alone, 5% to montelukast alone, and 55% to neither medication. Compared to those who responded to neither medication, favorable response to fluticasone alone was significantly (p < 0.05) associated with higher levels of exhaled nitric oxide (eNO), total eosinophilic count (TEC), serum IgE, serum eosinophilic cationic protein (ECP), and lower levels of methacholine PC20, pre-bronchodilator FEV1 percent predicted and FEV1/FVC; favorable response to montelukast alone was significantly associated with younger age and shorter duration of disease. Greater differential response to fluticasone over montelukast was associated with higher bronchodilator use, FEV1 response to bronchodilator, eNO, ECP, and lower pre-bronchodilator FEV1 percent predicted and FEV1/FVC, and methacholine PC20. Thus, responses to fluticasone and montelukast vary considerably in children with asthma. Children with lower pulmonary function or higher levels of markers associated with allergic inflammation should receive ICS therapy. Children without these indicators could receive a therapeutic trial of either ICS or LTRA. The major publication appeared in the Journal of Allergy and Clinical Immunology:
Szefler SJ, Phillips B, Martinez FD, Chinchilli VM, Lemanske RF, Jr, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005; 115:233-242. 15577824

