Summary:
In preschool children with (a) recurrent wheezing episodes, (b) positive Asthma Predictive Index (API), and (c) a severe wheezing exacerbation in the year prior to enrollment, the rate of wheezing/asthma exacerbations requiring systemic corticosteroids over a 12-month study period is equal for maintenance daily low-dose inhaled corticosteroids (ICS) when compared to intermittent high-dose ICS taken during respiratory tract illness for 7 days.
Design:
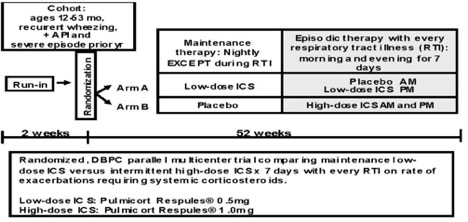
MIST is a randomized, double-blind, double-dummy, placebo-controlled, parallel, multicenter comparison of two strategies (maintenance daily low-dose ICS compared to intermittent high-dose ICS for 7 days during RTI) directed at reducing risk (exacerbations requiring systemic corticosteroids, primary outcome) and reducing impairment (episode-free days, severity of symptoms during RTI, albuterol use, and quality of life) in preschool children 12-47 months of age with the following phenotypic characteristics: recurrent wheezing, + API, and history of severe exacerbation in the year prior to enrollment. There will be a 2-week observation period to qualify and characterize the participants with respect to baseline demographic, atopic/asthma and genetic factors followed by a 52-week treatment phase. The rate of exacerbations that require system corticosteroids is the primary outcome.
Population:
Childhood Asthma Research and Education (CARE) Network
Link:
Enrollment for MIST began in August 2008 with a target sample size of 250 randomized children into the two treatment arms. The final patient visits occurred in July 2010. Daily was not significantly different from intermittent budesonide on frequency of exacerbations (P=0.6, relative rate 0.99, 95%CI, 0.71-1.35) nor on time to first (hazard ratio=0.97, 95%CI, 0.76-1.22, P=0.82) or second (hazard ratio=0.79, 95%CI, 0.49-1.32, P=0.38) exacerbation. Comparable efficacies (all P>0.05) were observed for rates of treatment failure and urgent/emergent visits for wheezing, severity of respiratory-tract illnesses, proportion of episode-free days, albuterol use, missed school/work, and changes in quality of life, exhaled nitric oxide levels, and growth. ICS exposure was 3.3-fold lower with intermittent compared to daily budesonide. The major publication appeared in NEJM:
Zeiger RS, Mauger D, Bacharier LB, Guilbert T, Martinez FD, Lemanske Jr RF, Strunk RC, Covar R, Szefler SJ, Boehmer S, Jackson DJ, Sorkness CA, Gern J, Kelly HW, Friedman NJ, Mellon MH, Schatz M, Morgan WJ, Chinchilli VM, Raissy HH, Bade E, Malka-Raise J, Beigelman A, Taussig LM for the Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Daily or Intermittent Budesonide in Preschool Children with Recurrent Wheezing. NEJM 2011; Vol. 365(20), Pages 1990-2001. PMID: 22111718 PMCID: PMC3247621.

