Summary:
In children aged 24-48 months, who are at high risk of developing asthma, a 24-month period of therapy with fluticasone will not influence the development of significant asthma. This will be assessed by the proportion of asthma-free days during the one-year period of observation, after termination of treatment.
Design:
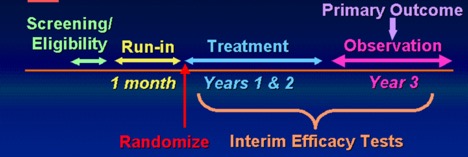
The PEAK trial was designed as a double-blind, randomized, placebo-controlled, parallel comparison of inhaled fluticasone to placebo in children 2 to 4 years of age who are at high risk of developing asthma. An eligible child must have exhibited a positive asthma predicted index (API), defined as follows: (1) more than three exacerbations of wheezing (each at least 24 hours in duration and at least one physician-confirmed) during the previous 12 months; (2) at least one major condition (parental history of asthma, MD-diagnosed atopic dermatitis, allergic sensitization to at least one aeroallergen) or at least two minor conditions (wheezing unrelated to colds, eosinophils above 4% in circulation, allergic sensitization to milk, egg, or peanuts). There was a 4-week run-in period to qualify and characterize children. Children were randomized (stratified by clinical center, age group, and gender) to active fluticasone or placebo. Children underwent a continuous treatment schedule for a period of 24 months, followed by an observation period of one year during which the main outcomes will be assessed. The primary outcome was the proportion of asthma-free days during the 1-year observation period that followed the 2-year treatment period.
Population
Childhood Asthma Research and Education (CARE) Network
Enrollment for PEAK began in January 2001 and the last patient visits occurred in October 2004. There were 285 randomized children (143 on ICS, 142 on placebo). During the observation year, the two groups were not significantly different in the proportion of episode-free days, number of exacerbations, or lung function. During the two-year treatment period, ICS was associated with a 4.8% greater proportion of episode-free days (means of 93.2% and 88.4%, p=0.006), and a 32.0% lower rate of exacerbations (mean events per 100 child-years of 57.4 and 89.4, p<0.001). The mean increase in height in the ICS-group was 1.1 cm less than the placebo group at 24 months (means of 12.6 cm and 13.7 cm, p<0.001). Compared to placebo, the ICS-group had a similar growth velocity during the last 12 months of treatment and a higher velocity during the observation-year. The major publication appeared in the New England Journal of Medicine: Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF Jr, Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Two year inhaled corticosteroid treatment on subsequent asthma in high-risk toddlers. NEJM 2006; 354:1985-1997. PMID: 16687711

